Aneurysm
Stay ahead of aneurysm risk
Small changes matter. Only deep clinical AI flags suspected aneurysms and tracks growth + morphology over time—empowering earlier, more informed care.
AI-driven aneurysm care, start to finish
Rapid Aneurysm helps you spot suspected cerebral aneurysms earlier, track progression with precision, and prioritize patients who need intervention.
Detect what others might miss
Detect what others might miss
Enhance your ability to uncover cerebral anomalies
Make prompt, confident decisions
Make prompt, confident decisions
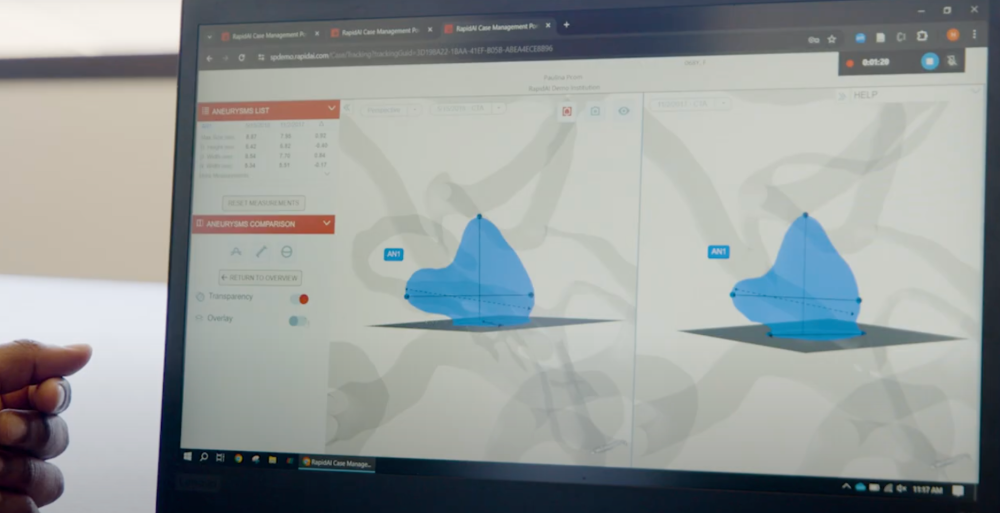
Convert 2D images to 3D models to precisely measure changes over time
Grow your practice
Grow your practice
Improve care and increase patient volume with advanced surveillance tools
Elevate your treatment planning
Elevate your treatment planning
Confidently track aneurysm progression + help patients grasp their condition
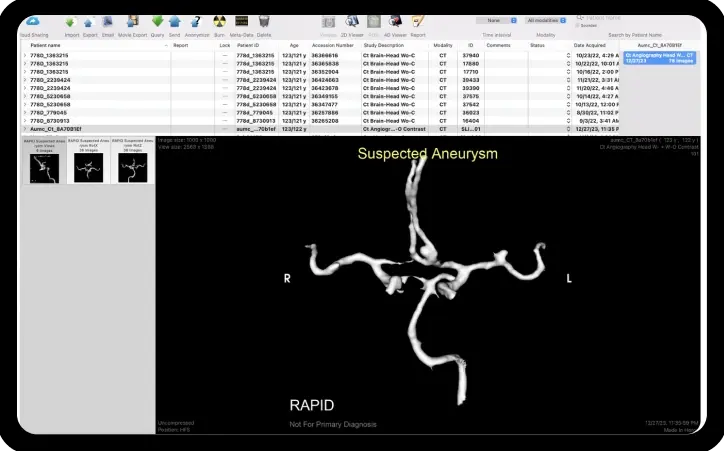
Diagnose more incidental cases
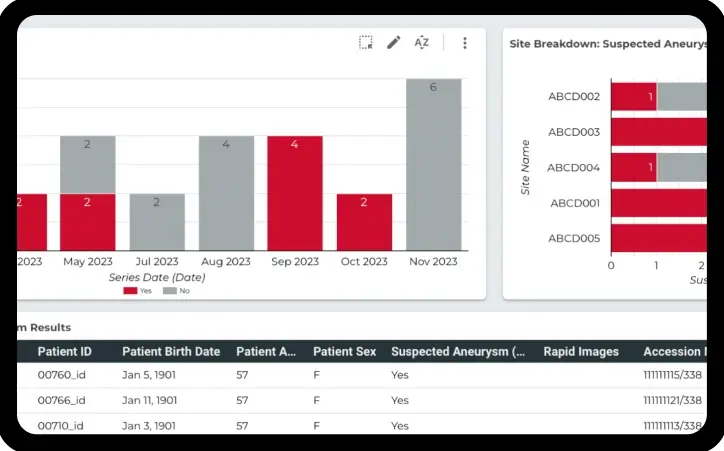
Reports for scheduling and identification of lost patients
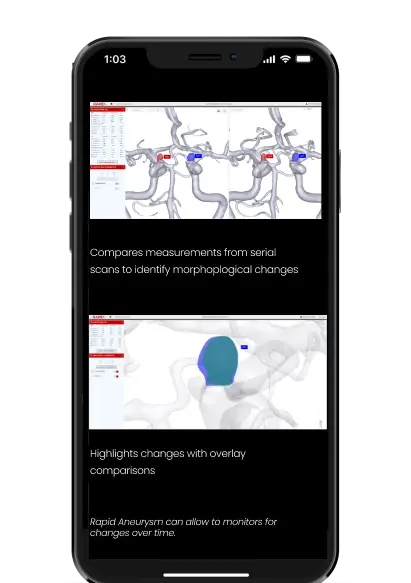
Accurate, efficient, consistent tracking
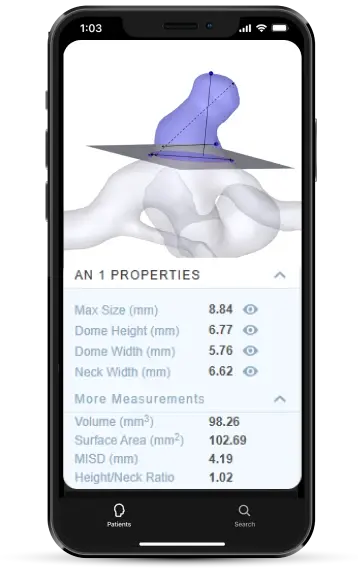
Detailed measurements and procedure planning
Elevate your program
Find
Get notified of suspected aneurysms throughout the network
Manage
Identify “lost” patients and see scheduling reports
Track
Highlight, visualize, + measure anomalies
Compare measurements + morphology for surveillance patients
Treat
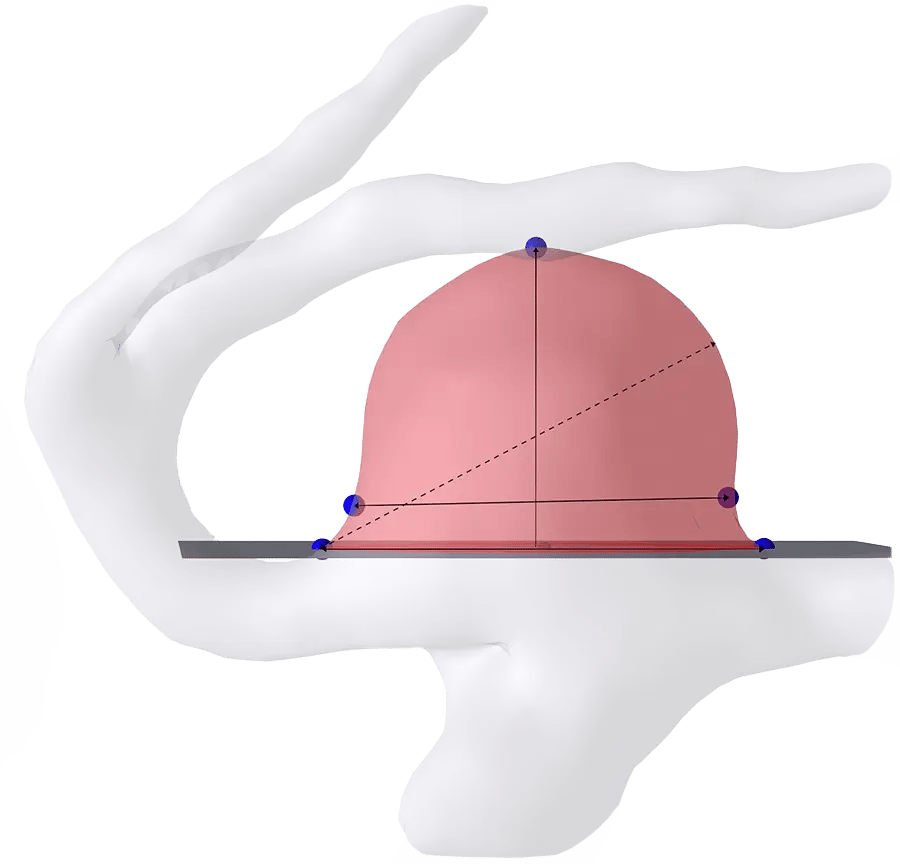
Plan procedures with detailed measurements like
-
Dome width + height
-
Neck width + height
-
Max size, surface area, + volume
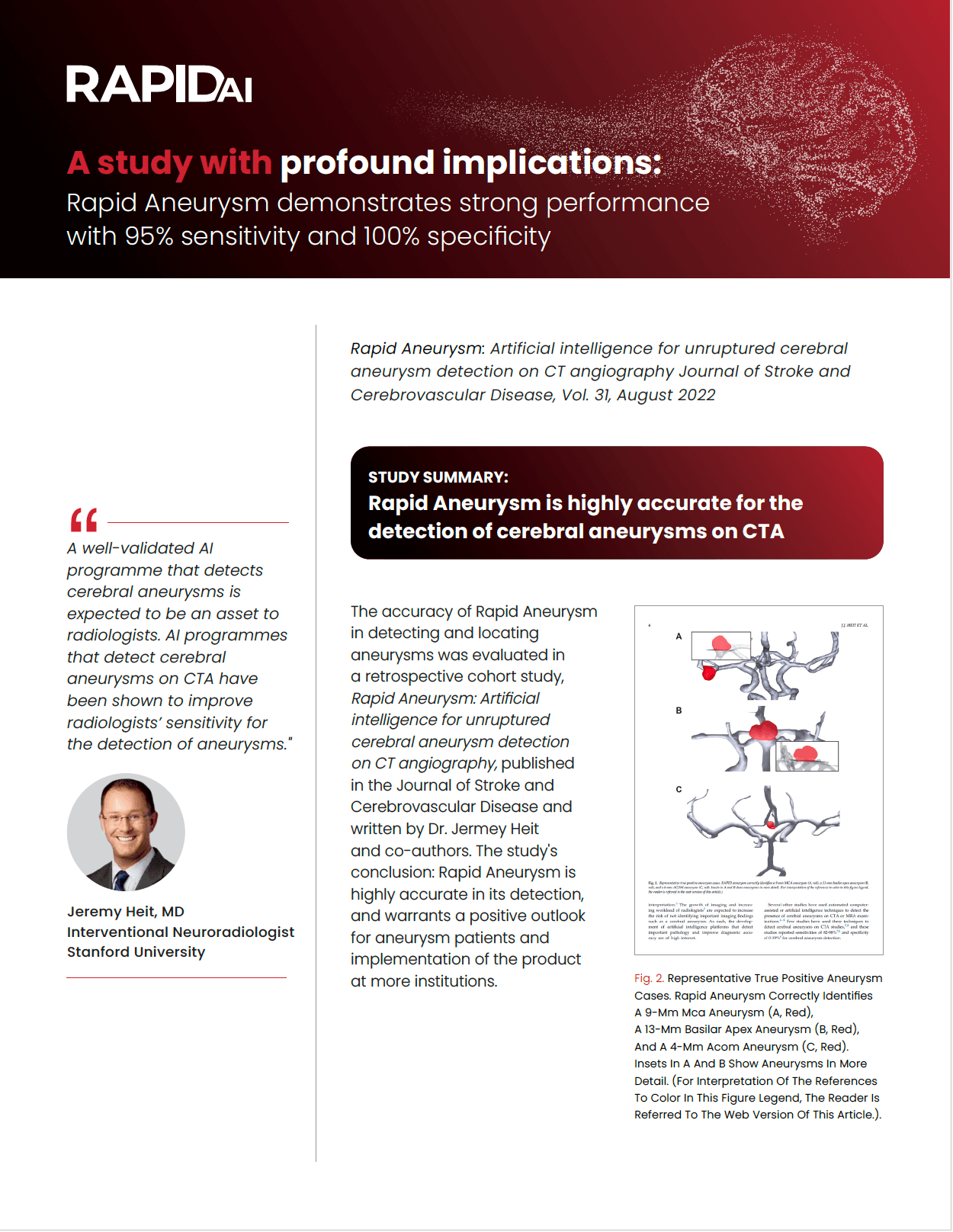
Sensitivity
Specificity
Identify aneurysms 4mm and above
Testimonials

Rapid Aneurysm has increased our detection of incidental cerebral aneurysms on imaging studies performed for other indications. It essentially functions as a “second pair of eyes” looking at all of our cross-sectional vascular imaging studies for aneurysms."
David Fiorella, MD
Director, Stony Brook Cerebrovascular Center, Co-Director, Stony Brook Cerebrovascular and Comprehensive Stroke Center

The AI-enabled aneurysm volume and surface area tool represents a revolution in technique for measuring aneurysm size and therefore growth."
Daniel Sahlein, MD
Director of Stroke at Ascension St. Vincent’s Indianapolis