Life Sciences
Support that sharpens evidence
Design and execute successful clinical trials with proven technology and tactics

%20(1).png)




Science and clinical validation are in our DNA
-
Our platform was used to select patients and conduct core lab analyses for some of the most influential stroke trials ever performed – all 6 of which are published in NEJM.
-
Our clinical rigor helped us change guidelines and establish our AI solutions as the most impactful in stroke care.
And we’re here to help you do the same.
We understand your challenges
Recruiting the most eligible patients
Study startup times
Integration with clinical workflows
High cost for sponsors
Clinical trials engineered for success

Strategic study design
Our team provides expert guidance in designing clinical trials, ensuring scientific rigor while integrating with clinical workflows. We help define precise eligibility criteria, setting you up for success from the start.

Smarter infrastructure & start-up
We streamline trial start-up by identifying experienced sites from our extensive network to ensure efficiency & performance.

Faster enrollment
Our post-scan eligibility notifications help optimize patient identification and enrollment, and our platform gives sponsors visibility into site screening.
Data analysis support
Our deep clinical AI solutions help reduce data variability across sites and streamline data transfer to vendors or sponsors.

Consistent execution
We simplify trial execution by supporting site training and managing complex study tasks. Our tools + services boost patient retention to ensure study continuity.
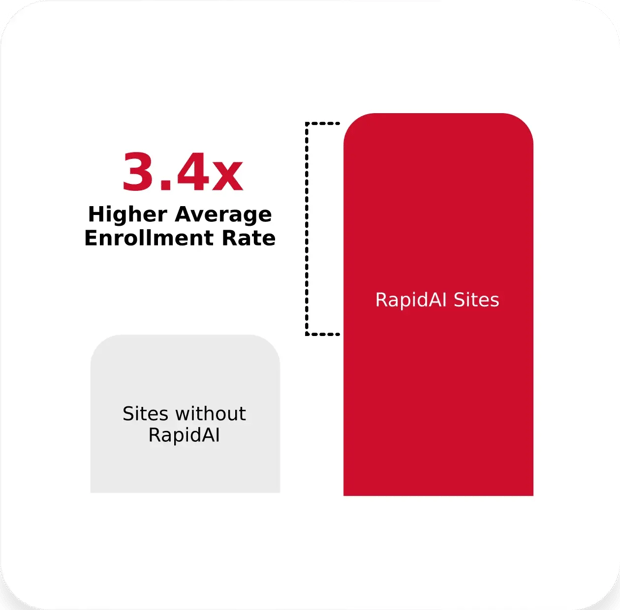
When data drive decisions, progress follows
In a multi-center (250 sites) international stroke trial that used our customized trial notifications, sites that enrolled at least one patient had a 3.4x higher average enrollment rate than sites without RapidAI.
Testimonials
We used Rapid in the EXTEND, EXTEND-IA and EXTEND-IA TNK randomized trials at over 20 sites across Australia and New Zealand. It's straightforward to install and works with the full range of CT and MR devices and PACS systems. The fully automated results are robust to common artefacts, easily interpretable and provide standardization across sites with a range of imaging experience."
Bruce Campbell, MBBS(Hons), BMedSc, PhD, FRACP
Professional Fellow, Department of Medicine, University of Melbourne and Head of Stroke, Royal Melbourne Hospital
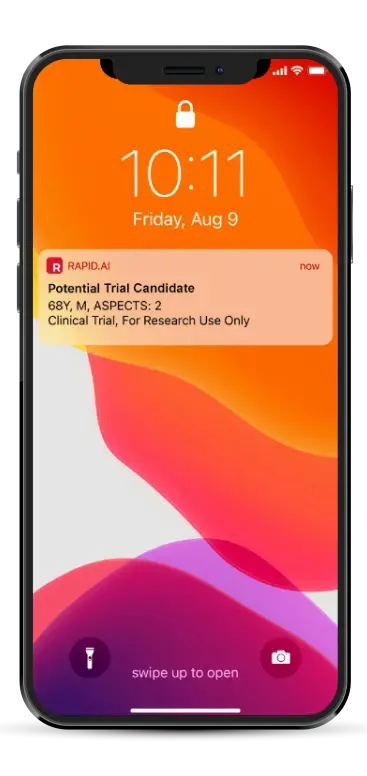
Notification triggered
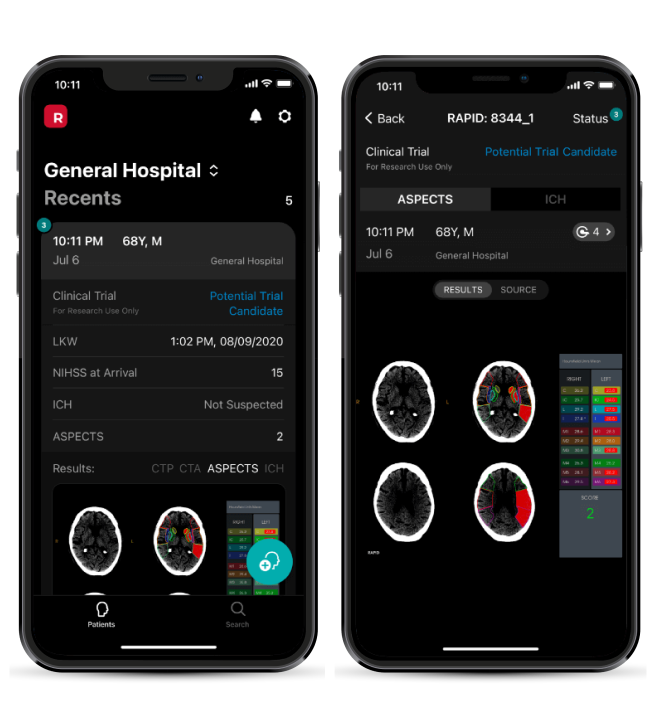
Patient flagged in the app
results & criteria viewable
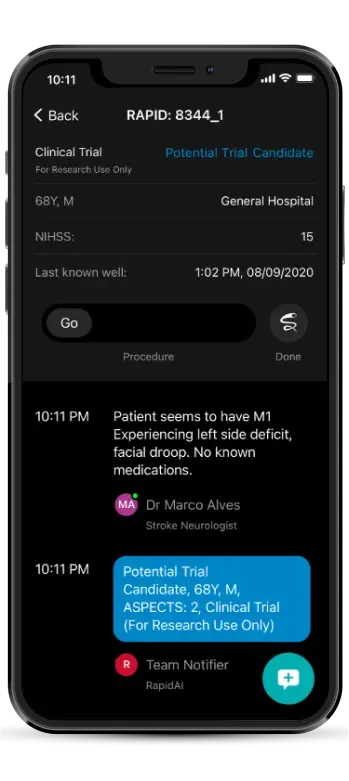
Team collaboration for enrollment


